Address from JPVAS leader

Welcome to the JPVAS website!
JPVAS is a central organization for vasculitis research in Japan, composed of members from multiple medical departments related to vasculitis. We are engaged in a variety of activities with the aim of contributing to the standardization and advancement of clinical and pathological diagnosis and treatment for refractory vasculitides.(Naoto Tamura, M.D., Ph.D, Department of Internal Medicine and Rheumatology, Juntendo University School of Medicine, Tokyo)
Mission of JPVAS
The Research Group for Improving Medical Standards and Patient Quality of Life for Intractable Vasculitis (Intractable Vasculitis Group) under the Intractable Disease Policy Research Project of the Ministry of Health, Labour and Welfare of Japan is working to improve medical standards and raise awareness about vasculitic diseases, with an all-Japan collaboration. The aim is to promote measures against intractable diseases and specified chronic pediatric diseases by serving as a command center for research and development promotion, thereby improving the medical standards for intractable vasculitis diseases and enhancing patients’ quality of life (QOL).
History of JPVAS
Since 1972, Japan’s Ministry of Health has organized research groups as a measure to investigate diseases of unknown cause and establish treatments. Research groups for rheumatoid arthritis with vasculitis, Takayasu arteritis, and Buerger’s disease were established. In 1976 these research groups merged to form the Systematic Vascular Lesions Research Group. Subsequently, in 1990, the name was changed to the Intractable Vasculitis Research Group, and its activities have continued.
| year | Principal Investigator | Name of the Research Group |
|---|---|---|
| 1972-1975 | Dr. Yuichi Shiokawa | RA with vasculitis |
| 1972-1975 | Dr. Kiyoshi Inada | Takayasu arteritis |
| 1972-1975 | Dr. Koichi Ishikawa | Berger’s disease |
| 1976-1978 | Dr. Yuichi Shiokawa | the Systematic Vascular Lesions Research Group |
| 1979-1984 | Dr. Yoshiro Fukuda | the Systematic Vascular Lesions Research Group |
| 1985-1989 | Dr. Yoshio Mishima | the Systematic Vascular Lesions Research Group |
| 1990-1992 | Dr. Tatsuzo Tanabe | the Intractable Vasculitis Research Group |
| 1993-1995 | Dr. Toshihiko Nagasawa | the Intractable Vasculitis Research Group |
| 1996-2001 | Dr. Hiroshi Hashimoto | the Intractable Vasculitis Research Group |
| 2002-2007 | Dr. Shoichi Ozaki | the Intractable Vasculitis Research Group |
| 2008-2013 | Dr. Hiroshi Makino | the Intractable Vasculitis Research Group |
| 2014-2016 | Dr. Yoshihiro Arimura | the Intractable Vasculitis Research Group |
| 2017-2022 | Dr. Masahiro Harigai | the Intractable Vasculitis Research Group |
| 2023- | Dr. Naoto Tamura | the Intractable Vasculitis Research Group |
Organization of JPVAS 2023-2025
6 subcommittees were established within the group, and each subcommittee chair implemented the third-year research topics.

Publication of JPVAS
Recent Topics from JPVAS
Circ J. 2025 Apr 25;89(5):612-619.
Haruhito Uchida, et al. with JPVAS.
Background: This study aimed to clarify recent clinical features and treatment outcomes in Japanese patients with newly diagnosed Takayasu arteritis (TAK) during the first 2 years of treatment.
Methods and results: A nationwide multicenter retrospective cohort study for TAK was implemented to collect data between 2007 and 2014. The primary outcome of the study was clinical remission at Week 24. Of the 184 participants registered, 129 patients with newly diagnosed TAK were analyzed: 84% were female and the mean age at onset was 35 years. Clinical symptoms at diagnosis were mostly associated with large-vessel lesions. Frequent sites of vascular involvement included the carotid artery, subclavian artery, aortic arch, and descending aorta. The mean initial dose of prednisolone administered was 0.68 mg/kg/day, and 59% and 17% of patients received immunosuppressive drugs and biologics, respectively, by Week 104. Clinical remission at Week 24 and sustained clinical remission with daily prednisolone at ≤10 mg at Week 52 were achieved in 107 (82.9%) and 51 (39.5%) patients, respectively. The presence of signs and symptoms linked to large-vessel lesions was associated with failure to achieve sustained clinical remission at Week 52.
Conclusions: We elucidated the clinical characteristics, treatment outcomes, and factors associated with failure to achieve sustained clinical remission in patients with newly diagnosed TAK in Japan during the first 2 years of treatment.
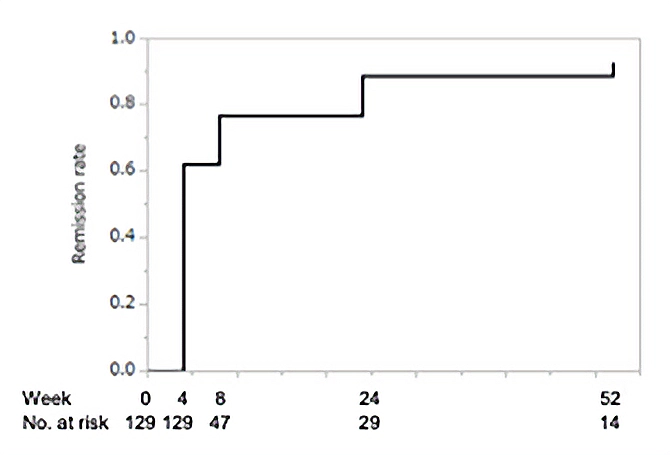
The cumulative clinical remission rate in overall patients (n=129) is shown. Clinical remission was defined as disappearance of all items of the disease activity domain of the draft core set of remission criteria by JPVAS
Annual activity summary of JPVAS from April 2024 to March 2025
Objectives: Serving as a pivotal institution for clinical practice and research advancement in intractable vasculitis, this study targets intractable vasculitides designated as intractable diseases and pediatric chronic specific diseases, promotes strategic initiatives for rare and pediatric chronic diseases, and contributes to enhancing medical standards and quality of life (QOL) for patients with intractable vasculitis. The principal aims encompass: 1) public education and knowledge dissemination regarding vasculitis, 2) revision of clinical practice guidelines (CPGs), 3) validation and investigation of vasculitis management, CPGs, diagnostic criteria, and disease severity classification, 4) facilitation of collaborative research initiatives supported by the Japan Agency for Medical Research and Development (AMED), and 5) advancement of international collaborative activities.
Methods: The entire research consortium promoted the Japan Vasculitis Study Group (JPVAS) prospective cohort study, while each of the five subcommittees, under the leadership of respective chairs, deliberated upon and executed both ongoing and novel research endeavors.
Results: Overview: Secondary research proposals utilizing clinical data and biospecimens from the JPVAS prospective cohort study were solicited, with the steering committee approving nine research projects. The first and second group meetings were convened in hybrid format on June 7 and December 20, 2024, respectively, to assess progress. The interdisciplinary subcommittee analyzed survey data concerning evidence-practice gaps in eosinophilic granulomatosis with polyangiitis (EGPA). The research consortium website underwent comprehensive revision, including development of an English version and updated disease descriptions. A joint symposium was conducted at the Japan College of Rheumatology annual meeting to disseminate current evidence in vasculitis management. The clinical pathology subcommittee sustained vasculitis pathology consultation services, reporting on 11 cases. A “Compendium of Staining Protocols for Vasculitis Pathology Diagnosis” was developed to standardize and disseminate diagnostic methodologies, scheduled for publication and online posting in 2025. The large vessel vasculitis clinical subcommittee conducted systematic reviews (SRs) by early-career investigators and formulated draft recommendations for CPG revision. Analysis of giant cell arteritis utilizing retrospective cohort data was published, with proposed diagnostic criteria under consideration; revised diagnostic criteria for Takayasu arteritis were similarly developed. Prospective study results examining clinical manifestations and remission rates in Takayasu arteritis were published. In collaboration with AMED-funded research groups, multicenter investigations of biomarkers for Takayasu arteritis-associated complications were advanced. Additional initiatives included analysis of surgical case registries, epidemiological investigations utilizing Medical Data Vision (MDV) database, and evaluation of tocilizumab indications in pediatric Takayasu arteritis. The medium and small vessel vasculitis clinical subcommittee initiated patient enrollment for a registry study (RemiTAVA) investigating avacopan, a C5a receptor inhibitor, in microscopic polyangiitis (MPA)/granulomatosis with polyangiitis (GPA) patients, targeting 400 participants with planned analysis of interstitial lung disease. The RemIRIT study results were analyzed and submitted for publication. Scoping reviews and SRs were conducted for EGPA guideline revision. Research consortium members contributed to the development of the 2025 Diagnosis and Treatment Guidelines for Interstitial Lung Disease Associated with Connective Tissue Diseases (joint initiative of the Japanese Respiratory Society and Japan College of Rheumatology). Analysis of nationwide epidemiological surveillance data for polyarteritis nodosa was advanced. Research protocols for developing peripheral neuropathy assessment scores and biomarker discovery were designed, with implementation planned for 2025. The international clinical research subcommittee participated in the Vasculitis Clinical Research Consortium (VCRC) Investigators Meeting for scientific exchange, promoting international collaborative studies including the Vasculitis Pregnancy Registry (VPREG) and AAV Patient-Reported Outcomes (AAV-PRO) Japanese translation project. The consortium participated in the 21st International Vasculitis Workshop 2025 (Barcelona) for presentation and discussion of research findings. Active engagement occurred at the inaugural meeting toward revision of the 2012 Chapel Hill Consensus Conference (CHCC2012) nomenclature for vasculitides. The consortium’s activities were presented at the Vasculitis Networks Collaborators Meeting.
Conclusions: Continued development and expansion of these research initiatives are anticipated to substantially contribute to advancing medical standards and improving QOL for patients with intractable vasculitis.







